Chemistry, 12.08.2020 04:01 perezsamantha3oqr0za
H2S(g) 2H2O(l)3H2(g) SO2(g) Using standard absolute entropies at 298K, calculate the entropy change for the system when 1.60 moles of H2S(g) react at standard conditions.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 23.06.2019 14:00, jamiahfernandes14
What can happen to an atoms electrons when an electric current is passed through the atom?
Answers: 1

Chemistry, 23.06.2019 15:00, Fangflora3
What do we call the rows on the periodic table? a. periodb. familyc. groupd. metals
Answers: 1
You know the right answer?
H2S(g) 2H2O(l)3H2(g) SO2(g) Using standard absolute entropies at 298K, calculate the entropy change...
Questions in other subjects:


Chemistry, 31.07.2019 10:30




Physics, 31.07.2019 10:30

Geography, 31.07.2019 10:30

English, 31.07.2019 10:30


History, 31.07.2019 10:30

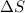 = 473.92J/K.mol
= 473.92J/K.mol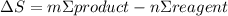

 :
: