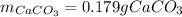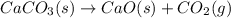Chemistry, 12.08.2020 06:01 DASASDAEDWEDA
Determine the mass of CaCO3 required to produce 40.0 mL CO2 at STP. Hint use molar volume of an ideal gas (22.4 L)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1


Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 23.06.2019 01:50, UncleVictor5188
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
You know the right answer?
Determine the mass of CaCO3 required to produce 40.0 mL CO2 at STP. Hint use molar volume of an idea...
Questions in other subjects:

Social Studies, 18.05.2021 19:10





History, 18.05.2021 19:10