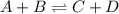Chemistry, 12.08.2020 07:01 JFrocks2480
In a reversible reaction, the endothermic reaction absorbs the exothermic reaction releases. A. less energy than B. None of these, endothermic reactions release energy C. the same amount of energy as D. more energy than

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:30, allofthosefruit
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3


Chemistry, 22.06.2019 23:30, sweaversw9602
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
In a reversible reaction, the endothermic reaction absorbs the exothermic reaction releases. A. les...
Questions in other subjects:

Mathematics, 19.03.2021 16:00