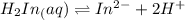Chemistry, 12.08.2020 06:01 qwemnb7401
PLEASE HELP!! What direction do you predict the addition of a base to the solution containing bromophenol blue will drive the equilibrium? Explain your prediction in terms of Le Châtelier's principle.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:50, mi364
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3



Chemistry, 22.06.2019 22:00, genyjoannerubiera
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
You know the right answer?
PLEASE HELP!! What direction do you predict the addition of a base to the solution containing bromop...
Questions in other subjects:



Biology, 28.08.2019 12:30

Biology, 28.08.2019 12:30

English, 28.08.2019 12:30



Social Studies, 28.08.2019 12:30


Mathematics, 28.08.2019 12:30