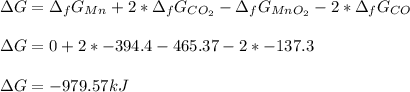Chemistry, 04.08.2020 16:01 patricklcc07777
Calculate Delta G for each reaction using Delta Gf values: answer kJ ...thank you
a) H2(g)+I2(s)--->2HI(g)
b) MnO2(s)+2CO(g)--->Mn(s)+2CO2(g)
c) NH4Cl(s)--->NH3(g)+HCl(g)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1


Chemistry, 22.06.2019 12:30, gonzalesalexiaouv1bg
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3
You know the right answer?
Calculate Delta G for each reaction using Delta Gf values: answer kJ ...thank you
a) H2(g)+I2(s)---...
Questions in other subjects:


Mathematics, 06.02.2022 02:40



Business, 06.02.2022 02:50


Mathematics, 06.02.2022 02:50