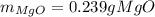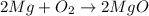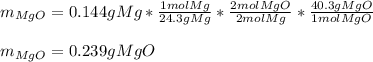Chemistry, 04.08.2020 14:01 denisebaslee15
· A 0.100g sample of Mg when combined with O2 yields 0.166g of Mgo, a
second Mg sample with a mass of 0.144g is also combined with O2. What
mass of MgO is produced from the second sample?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 22:30, arodavoarodavo
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 00:20, cmflores3245
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3

Chemistry, 23.06.2019 01:00, zitterkoph
Which of the following is a physical change? a. burning a piece of wood b. sawing a piece of wood in half c. rust forming on an iron fence d. a copper roof changing color from orange to green
Answers: 1
You know the right answer?
· A 0.100g sample of Mg when combined with O2 yields 0.166g of Mgo, a
second Mg sample with a mass...
Questions in other subjects:


Mathematics, 04.06.2020 17:58

Mathematics, 04.06.2020 17:58

Mathematics, 04.06.2020 17:58





Social Studies, 04.06.2020 17:58