

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, anggar20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1


Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
You know the right answer?
How many grams of dipotassium succinate trihydrate (K2C4H4O4·3H2O, MW = 248.32 g/mol) must be added...
Questions in other subjects:

Health, 28.07.2019 00:50


Biology, 28.07.2019 00:50


Biology, 28.07.2019 00:50


Biology, 28.07.2019 00:50

Biology, 28.07.2019 00:50

Spanish, 28.07.2019 00:50

Biology, 28.07.2019 00:50

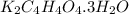 = 660.mL
= 660.mL![PH =pKa + log\dfrac{[Salt]}{[Acid]}](/tpl/images/0716/8416/1a967.png)
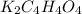 = 660.mL
= 660.mL Volume (liters)
Volume (liters)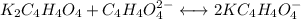
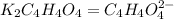
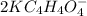 = 2 × 0.038082 mol
= 2 × 0.038082 mol![pH =pKa + log\dfrac{[Salt]}{[Acid]}](/tpl/images/0716/8416/edfb4.png)
![pH =pKa_2 + log\dfrac{[K_2C_4H_4O_4]}{[KC_4H_4O_4^-]}](/tpl/images/0716/8416/55335.png)
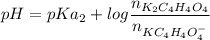
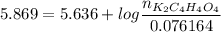
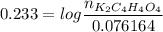
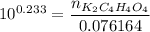
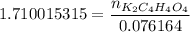
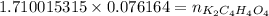
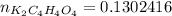
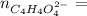 ( 0.1302416 + 0.038082) mol
( 0.1302416 + 0.038082) mol

