
Chemistry, 02.08.2020 01:01 amandasantiago2001
Which of the following statements is true? a) The rate constant does not depend on the activation energy for a reaction where the products are lower than the reactants. b) A catalyst raises the activation energy of a reaction. c) Rate constants are temperature dependent.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 11:30, claudr03
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3
You know the right answer?
Which of the following statements is true? a) The rate constant does not depend on the activation en...
Questions in other subjects:

Advanced Placement (AP), 25.02.2020 06:21

Geography, 25.02.2020 06:21



Mathematics, 25.02.2020 06:21


Mathematics, 25.02.2020 06:21



![r = k(T) [A]^{m}[B]^{n}](/tpl/images/0716/5763/a0ca9.png)
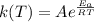
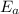 = Activation energy
= Activation energy

