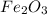Chemistry, 01.08.2020 16:01 bethanybowers4986
1) If there are 0.375 moles 15 grams of compound, what would its molar mass be?
2) How many moles are there in 90 grams of magnesium oxide?
3) Calculate the molecular mass of ammonia.
4) Calculate the formula mass of sodium hydrogen sulphate.
5) Assuming the relation atomic mass of metal M to be 56, what would the empirical formula of its oxide containing 70.0% of M be?
I want you to answer all of the questions, 5 of them
And don't answer the question just for points if you don't know what it means
And also show workings I won't accept an answer without an explanation

Answers: 1


Other questions on the subject: Chemistry




You know the right answer?
1) If there are 0.375 moles 15 grams of compound, what would its molar mass be?
2) How many mol...
2) How many mol...
Questions in other subjects:

Mathematics, 23.07.2020 17:01

Computers and Technology, 23.07.2020 17:01

Biology, 23.07.2020 17:01

Mathematics, 23.07.2020 17:01


Chemistry, 23.07.2020 18:01

Mathematics, 23.07.2020 18:01



History, 23.07.2020 18:01



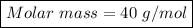
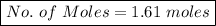

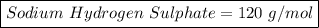

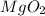
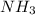

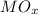 (Metal oxide) = 56 / 70 * 100
(Metal oxide) = 56 / 70 * 100