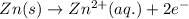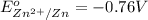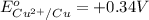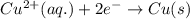Chemistry, 01.08.2020 08:01 sayedabdullah
Which reaction occurs at the anode of a galvanic cell that has a zinc
electrode in an electrolyte with zinc ions and a copper electrode in an
electrolyte with copper ions? The reduction potential for the reduction of Cu2+
= 0.34 V. The reduction potential for the reduction of Zn2+ = -0.76 V.
A. Zn(s) → Zn2+(aq) + 2e
B. Cu(s) → Cu2+(aq) + 2e
C. Zn2+ (aq) + 2e → Zn(s)
D. Cu2+ (aq) + 2e →
Cu(s)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, I1YBYY
Infants born with severe respiratory problems are sometimes given liquid ventilation: they breathe a liquid that can dissolve more oxygen than air can hold. one of these liquids is a fluorinated compound, cf3(cf2)7br. the solubility of oxygen in this liquid is 66 mlo2 per 100 ml liquid. in contrast, air is 21 % oxygen by volume. calculate the moles of o2 present in an infant's lungs (volume: 12 ml ) if the infant takes a full breath of air. assume a pressure of 1 atm in the lungs.
Answers: 1

Chemistry, 21.06.2019 13:20, valencial0917
Why is an elements atomic mass not listed as a whole number on the periodic table
Answers: 2

Chemistry, 21.06.2019 22:00, juansantos7b
Which of the following statements is true about planck’s law
Answers: 1

You know the right answer?
Which reaction occurs at the anode of a galvanic cell that has a zinc
electrode in an electrolyte w...
Questions in other subjects:

Mathematics, 19.11.2020 18:20






Chemistry, 19.11.2020 18:20

Biology, 19.11.2020 18:20


Social Studies, 19.11.2020 18:20