
Chemistry, 31.07.2020 19:01 lolomgwtfnvm4
A chemist studies the reaction below. 2NO(g) + Cl2(g) 2NOCl(g) He performs three experiments using different concentrations and measures the initial reaction rates (The data from the three experiments is in the table). 1. Write the rate law 2. Solve for k.
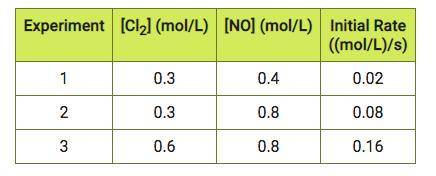

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, birdman2540
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2

Chemistry, 23.06.2019 01:30, yarrito20011307
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
A chemist studies the reaction below. 2NO(g) + Cl2(g) 2NOCl(g) He performs three experiments using d...
Questions in other subjects:


Mathematics, 06.06.2020 00:02

Biology, 06.06.2020 00:02

History, 06.06.2020 00:02

English, 06.06.2020 00:02

Biology, 06.06.2020 00:02

History, 06.06.2020 00:02

History, 06.06.2020 00:02

History, 06.06.2020 00:02

History, 06.06.2020 00:02

![Rate =k [NO]^{2}[Cl_{2}]](/tpl/images/0716/1051/5657a.png)
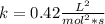
![Rate =k [NO]^{m}[Cl_{2}]^{n}](/tpl/images/0716/1051/72196.png)
![Rate1 = k[0.4]^{m}[0.3]^{n}=0.02\\Rate 2=k [0.8]^{m}[0.3}]^{n}=0.08\\\\\frac{Rate1}{Rate2}=\frac{0.02}{0.08} =\frac{k[0.4]^{m}[0.3]^{n}}{k[0.8]^{m}[0.3]^n}} \\\\\frac{1}{4} =(\frac{1}{2} )^{m},\\m=2](/tpl/images/0716/1051/97f3f.png)
![Rate3 =k [0.8]^{m}[0.6]^{n}=0.16\\Rate 2= k[0.8]^{m}[0.3}]^{n}=0.08\\\\\frac{Rate3}{Rate2}=\frac{0.16}{0.08} =\frac{k[0.8]^{m}[0.6]^{n}}{k[0.8]^{m}[0.3]^n}} \\\\\frac{2}{1} =(\frac{2}{1} )^{n},\\n=1](/tpl/images/0716/1051/2721a.png)
![Rate =k [NO]^{2}[Cl_{2}]^{1}](/tpl/images/0716/1051/cae2c.png)
![Rate =k [NO]^{2}[Cl_{2}]^{1}\\Rate 1=k [0.4]^{2}[0.3]^{1} =0.02\\k*0.16*0.3=0.02\\k=\frac{0.02}{0.16*0.3}=\frac{1}{8*(\frac{3}{10} )}=\frac{5}{12} = 0.42 \frac{L^{2}}{mol^{2}*s}](/tpl/images/0716/1051/f8211.png)


