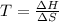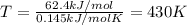Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1


Chemistry, 23.06.2019 06:00, irenecupcake4348
What does it mean for something to be dissolved in watera- it is submerged in water moleculesb-it is stirred in the water moleculesc- it is surrounded by water molecules d-it has water molecules added to it
Answers: 2
You know the right answer?
Use the reaction I2(s) I2(g), H = 62.4 kJ/mol, S = 0.145 kJ/(molK)
At what temperature is the react...
Questions in other subjects:

English, 10.01.2021 01:10

Mathematics, 10.01.2021 01:10


History, 10.01.2021 01:10


Mathematics, 10.01.2021 01:10


Spanish, 10.01.2021 01:10

History, 10.01.2021 01:10

Mathematics, 10.01.2021 01:10

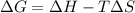
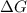 = Gibbs free energy
= Gibbs free energy 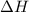 = enthalpy change = +62.4 kJ/mol
= enthalpy change = +62.4 kJ/mol 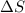 = entropy change = +0.145 kJ/molK
= entropy change = +0.145 kJ/molK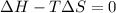 for reaction to be spontaneous
for reaction to be spontaneous