
Chemistry, 30.07.2020 23:01 JGottem6489
A certain reaction has the following general form. aA → bB At a particular temperature and [A]0 = 2.80 ✕ 10−3 M, concentration versus time data were collected for this reaction, and a plot of 1/[A] versus time resulted in a straight line with a slope value of +3.40 ✕ 10−2 L mol−1 s−1. (a) Determine the rate law, the integrated rate law, and the value of the rate constant for this reaction. (Rate expressions take the general form: rate = k . [A]a . [B]b.) rate law:

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, shamayajohnsonsh5
One of the cell membrane's functions is to protect the cell keep wastes in the cell create new cells keep light out of the cell
Answers: 1


Chemistry, 21.06.2019 23:30, hellokitty1647
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i. e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 05:30, saleenhernandez83
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1
You know the right answer?
A certain reaction has the following general form. aA → bB At a particular temperature and [A]0 = 2....
Questions in other subjects:


Mathematics, 22.07.2021 07:50

English, 22.07.2021 07:50


Medicine, 22.07.2021 07:50



Mathematics, 22.07.2021 07:50

Mathematics, 22.07.2021 07:50

Biology, 22.07.2021 07:50

![r=k[A]^2](/tpl/images/0715/7128/d4e0e.png)
![\frac{1}{[A]}=kt+ \frac{1}{[A]_0}](/tpl/images/0715/7128/acbfc.png)
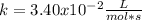
![\frac{d[A]}{dt}=-k[A]^2](/tpl/images/0715/7128/47b67.png)


