
How many moles are in 10.23 g of PO4-3? How many moles are 8.25 x 10^28 molecules of Na2CO3? What is the mass of 6 moles of CH2O? How many formula units are in 6.34 g of NaCl? How many ions are in 0.25 moles of Cu+2? How many grams are in 3.4 x 10^24 molecules of CH4? How many moles are in 10 mL of water (density of water = 1 g/mL)? (sorry for so many questions in one)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 22:10, zwbaby3693
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
How many moles are in 10.23 g of PO4-3? How many moles are 8.25 x 10^28 molecules of Na2CO3? What is...
Questions in other subjects:

History, 25.08.2019 19:20

Mathematics, 25.08.2019 19:20



Mathematics, 25.08.2019 19:20


History, 25.08.2019 19:20


History, 25.08.2019 19:20

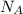 = 6.02 × 10²³ molecules
= 6.02 × 10²³ molecules


