Chemistry, 30.07.2020 03:01 christinafish9303
Aspirin (C9H8O4) is produced by the reaction of salicylic acid (C7H6O3, Molar mass = 138.1 g/mol) and acetic anhydride (C4H6O3, Molar mass = 102.1 g/mol) based on the BALANCED equation : C7H6O3(s) + C4H6O3(l ) → C9H8O4(s) + C2H4O2( l) If 63.07 grams of aspirin (Molar mass = 180.2 g/mol) was collected from an experiment when 138.1 grams C7H6O3 reacted with excess C4H6O3, what was the percent yield?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 19:30, Karinaccccc
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 23.06.2019 01:00, kaykardash
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2
You know the right answer?
Aspirin (C9H8O4) is produced by the reaction of salicylic acid (C7H6O3, Molar mass = 138.1 g/mol) an...
Questions in other subjects:








History, 14.07.2019 21:30


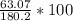 hould be used up in the reaction, yielding 180.2 g of C9H8O4. ⇒ Theoretical yield = 180.2g
hould be used up in the reaction, yielding 180.2 g of C9H8O4. ⇒ Theoretical yield = 180.2g