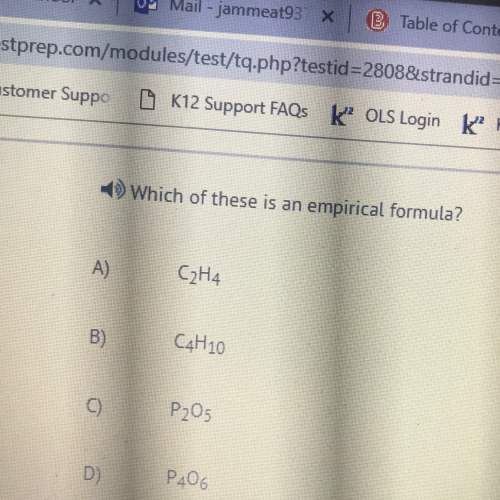
What may be expected when K < 1.0? Choose the THREE correct statements. The concentration of one or more of the reactants is small. The concentration of one or more of the products is small. The reaction will not proceed very far to the right. The reaction will generally form more reactants than products.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:50, stodd9503
Your roll: experienced electron speech is adressed to: a new "freshman class" of electrons job: write a speech task: you are to pretend that you are giving a speech to a new group of electrons. be sure to mention their placement in an atom, their charge, and their role in chemical bonding (ionic and covalent) be specific!
Answers: 3


Chemistry, 21.06.2019 18:50, ayoismeisjjjjuan
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x. xx ml
Answers: 1

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1
You know the right answer?
What may be expected when K < 1.0? Choose the THREE correct statements. The concentration of one...
Questions in other subjects:

Mathematics, 24.04.2020 05:38


Social Studies, 24.04.2020 05:39


History, 24.04.2020 05:39



Mathematics, 24.04.2020 05:39

Mathematics, 24.04.2020 05:39