
Chemistry, 29.07.2020 20:01 lerasteidl
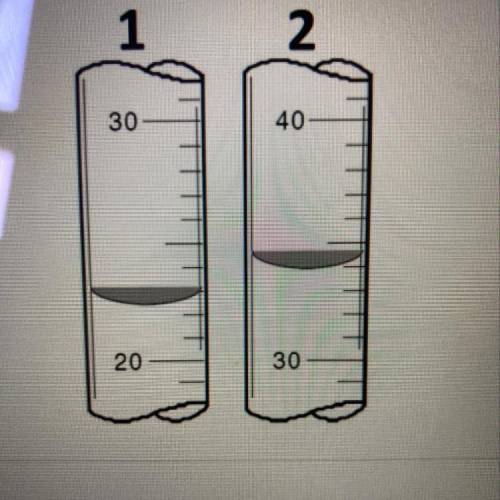
A titration experiement, showed Initial (2) and Final (1) readings, as seen displayed.
Determine the initial and final readings, then calculate how many mL of liquid was dispensed.
A) 9.50 cm
B) 5.50 cm
C) 11.5 cm
D) 10.05 cm

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, jamccoy3335
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 18:10, NEONREDBLADE
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
A titration experiement, showed Initial (2) and Final (1) readings, as seen displayed.
Determine th...
Questions in other subjects:



History, 07.09.2020 18:01

Biology, 07.09.2020 18:01

English, 07.09.2020 18:01

Mathematics, 07.09.2020 18:01

History, 07.09.2020 18:01

Mathematics, 07.09.2020 18:01

Mathematics, 07.09.2020 18:01



