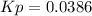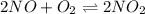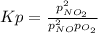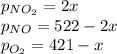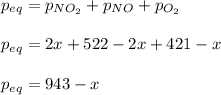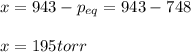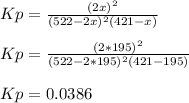Chemistry, 26.07.2020 14:01 jetblackcap
A reaction mixture at 175 K initially contains 522 torr of NO and 421 torr of O2. At equilibrium, the total pressure in the reaction mixture is 748 torr. Calculate Kp at this temperature. Express your answer to three significant figures.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bobbycisar1205
How does a hydroelectric power plant converts energy into energy.
Answers: 1



Chemistry, 23.06.2019 00:00, kittenalexis68
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
A reaction mixture at 175 K initially contains 522 torr of NO and 421 torr of O2. At equilibrium, th...
Questions in other subjects:

Social Studies, 13.05.2021 23:10



History, 13.05.2021 23:10

Mathematics, 13.05.2021 23:10

Mathematics, 13.05.2021 23:10

Spanish, 13.05.2021 23:10

History, 13.05.2021 23:10

Mathematics, 13.05.2021 23:10