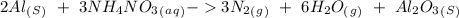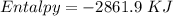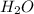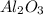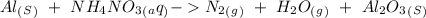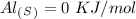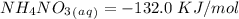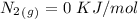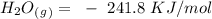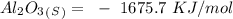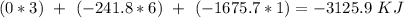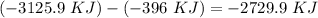Chemistry, 26.07.2020 14:01 babyquinnz
15. Ammonium nitrate, NH4NO3, and aluminum powder react explosively producing nitrogen gas, water vapor and aluminum oxide. Write the balanced equation and calculate the enthalpy change for this reaction.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2

Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
15. Ammonium nitrate, NH4NO3, and aluminum powder react explosively producing nitrogen gas, water va...
Questions in other subjects:

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

History, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01