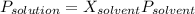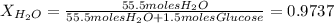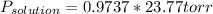Chemistry, 27.07.2020 01:01 wolfking800
The vapor pressure of pure water at 250C is 23.77 torr. What is the vapor pressure of water above a solution that is 1.500 m glucose, C6H12O6?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:00, ambarpena14
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 23:00, Mynameismath
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
You know the right answer?
The vapor pressure of pure water at 250C is 23.77 torr. What is the vapor pressure of water above a...
Questions in other subjects:


Mathematics, 04.05.2021 17:20




Mathematics, 04.05.2021 17:20

Mathematics, 04.05.2021 17:20