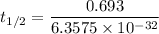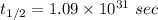Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, hannahmr092402
One of the following properties was originally used to arrange elements on the periodic table, but is no longer used to organize the modern version. which property fits this description?
Answers: 3


Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
The activation energy (E*) for 2N2O ---> 2N2 + O2 is 250 KJ. If the k for this reaction is 0.380/...
Questions in other subjects:

Mathematics, 13.09.2020 14:01

Mathematics, 13.09.2020 14:01

Mathematics, 13.09.2020 14:01

Physics, 13.09.2020 14:01

English, 13.09.2020 14:01

Mathematics, 13.09.2020 14:01

Biology, 13.09.2020 14:01

History, 13.09.2020 14:01

Mathematics, 13.09.2020 14:01

Mathematics, 13.09.2020 14:01

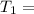 1001 K
1001 K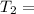 298 K
298 K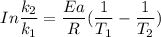
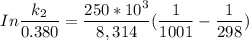
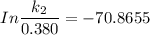

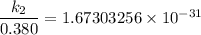
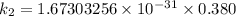
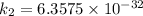 /M .sec
/M .sec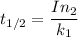
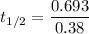
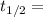 1.82368 secc
1.82368 secc