
Chemistry, 27.07.2020 01:01 ayoismeisjuam
Calculate the [H+] and pH of a 0.0040 M hydrazoic acid solution. Keep in mind that the Ka of hydrazoic acid is 2.20×10−5. Use the method of successive approximations in your calculations or the quadratic formula.

Answers: 3


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
Calculate the [H+] and pH of a 0.0040 M hydrazoic acid solution. Keep in mind that the Ka of hydrazo...
Questions in other subjects:






Biology, 20.10.2020 14:01

Social Studies, 20.10.2020 14:01

Geography, 20.10.2020 14:01


English, 20.10.2020 14:01

![[H^+]=0.000285](/tpl/images/0713/5320/5d625.png)

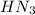 ). So:
). So:
![Ka=\frac{[H^+][N_3^-]}{[HN_3]}](/tpl/images/0713/5320/574a9.png)
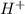 produced we will have 1 mol of
produced we will have 1 mol of  . So, we can use "X" for the unknown values and replace in the Ka equation:
. So, we can use "X" for the unknown values and replace in the Ka equation:![Ka=\frac{X*X}{[HN_3]}](/tpl/images/0713/5320/c6a9a.png)




![pH=-Log[H^+]=-Log[0.000285]=3.55](/tpl/images/0713/5320/8cdec.png)


