Chemistry, 25.07.2020 06:01 NikolaiSolokov
You are given 10.00 mL of a solution of an unknown acid. The pH of this solution is exactly 2.18. You determine that the concentration of the unknown acid was 0.2230 M. You also determined that the acid was monoprotic (HA). What is the pKa of your unknown acid

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, pleasehelpmeonthis
The difference between atomic mass and molar mass
Answers: 3

Chemistry, 21.06.2019 22:00, NREYESLDS2806
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1


You know the right answer?
You are given 10.00 mL of a solution of an unknown acid. The pH of this solution is exactly 2.18. Yo...
Questions in other subjects:










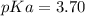
![pH=-log([H^+])\\](/tpl/images/0712/8744/a9ef9.png)
![[H^+]=10^{-pH}=10^{-2.18}=6.61x10^{-3}M](/tpl/images/0712/8744/456e0.png)
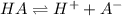
![Ka=\frac{[H^+][A^-]}{[HA]}](/tpl/images/0712/8744/39962.png)
![[HA]=0.2230M-6.61x10^{-3}M=0.2164M](/tpl/images/0712/8744/1a456.png)
 ). Thereby, the acid dissociation constant turns out:
). Thereby, the acid dissociation constant turns out: