Chemistry, 25.07.2020 03:01 bryanmcmillianjr
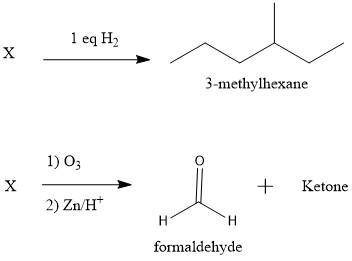
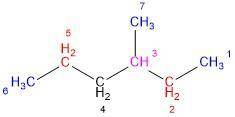
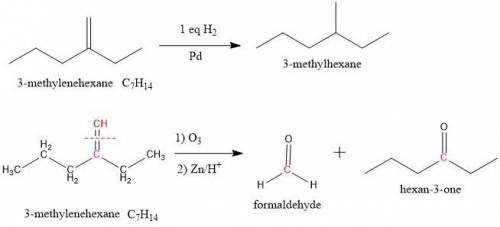
Compound X has the formula C7H14. X reacts with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 3-methylhexane. Treatment of X with ozone follwed by zinc in aqueous acid gives a ketone plus formaldehyde (CH2=O). What is the structure of X?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 14:50, rebeccamckellpidge
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
Compound X has the formula C7H14. X reacts with one molar equivalent of hydrogen in the presence of...
Questions in other subjects:

Mathematics, 18.03.2021 01:30

History, 18.03.2021 01:30

Spanish, 18.03.2021 01:30

Computers and Technology, 18.03.2021 01:30


Biology, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30



Mathematics, 18.03.2021 01:30

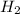 is added to the unknown molecule. This indicates that we only have 1 double bond in the molecule. Now, the next question is where is placed the double bond?
is added to the unknown molecule. This indicates that we only have 1 double bond in the molecule. Now, the next question is where is placed the double bond?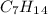 and the reactions. (See figure 3)
and the reactions. (See figure 3)