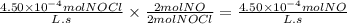Chemistry, 25.07.2020 03:01 damienwoodlin6
Nitrogen monoxide reacts with chlorine at high temperature according to the equation, 2 NO(g) + Cl2(g) → 2 NOCl(g) In a certain reaction mixture the rate of formation of NOCl(g) was found to be 4.50 x 10‑4 mol L‑1 s‑1. What is the rate of consumption of NO(g)?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, badgirl2005
This is a mixture that has the same composition throughout.
Answers: 1

Chemistry, 22.06.2019 00:30, rscott2649
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 02:30, drivinghydra
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 05:30, livigrace9004
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2
You know the right answer?
Nitrogen monoxide reacts with chlorine at high temperature according to the equation, 2 NO(g) + Cl2(...
Questions in other subjects:

Mathematics, 13.12.2021 04:10


History, 13.12.2021 04:10


Physics, 13.12.2021 04:20


Social Studies, 13.12.2021 04:20


Mathematics, 13.12.2021 04:20