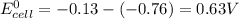Chemistry, 24.07.2020 22:01 bellagracebulle8018
Pb2+(aq) + 2 e- --> Pb(s) Eo = -0.13V Zn2+(aq) + 2 e- --> Zn(s) Eo = -0.76V 9. Given the half-cell potentials above, when the reaction Zn(s) + Pb2+(aq) --> Zn2+(aq) + Pb(s) is made into a voltaic cell, the Ecell is: A. 0.63 V B. -0.63 V C. 0.89 D. 0.89

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, alejandra1201
10. according to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants?
Answers: 3

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 21:40, k3rbycalilung
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2
You know the right answer?
Pb2+(aq) + 2 e- --> Pb(s) Eo = -0.13V Zn2+(aq) + 2 e- --> Zn(s) Eo = -0.76V 9. Given the half-...
Questions in other subjects:



Spanish, 10.06.2021 19:20



Mathematics, 10.06.2021 19:20

Mathematics, 10.06.2021 19:20



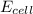 is 0.63 V
is 0.63 V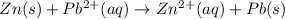
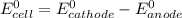
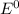 are standard reduction potentials.
are standard reduction potentials.![E^0_{[Pb^{2+}/Pb]}= -0.13V](/tpl/images/0712/6436/bacf1.png)
![E^0_{[Zn^{2+}/Zn]}=-0.76V](/tpl/images/0712/6436/4cd18.png)
![E^0_{cell}=E^0_{[Pb^{2+}/Pb]}- E^0_{[Zn^{2+}/Zn]}](/tpl/images/0712/6436/22253.png)