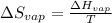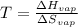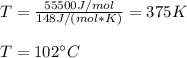Chemistry, 24.07.2020 20:01 disneyshree9427
The heat of vaporization of 1-pentanol is 55.5 kJ/mol, and its entropy of vaporization is 148 J/K. mol. What is the approximate boiling point of 1-pentanol? 100 oC 375 oC 0 oC 25 oC

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 23:30, Xavier8247
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 07:00, kotetravels10
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1

Chemistry, 23.06.2019 15:30, Marliii363782
An isotope undergoes radioactive decay. the new isotope that forms has an atomic number fhat is 2 less than the original isotopes. which kind of decay has occured and how do you know
Answers: 2
You know the right answer?
The heat of vaporization of 1-pentanol is 55.5 kJ/mol, and its entropy of vaporization is 148 J/K. m...
Questions in other subjects:


English, 14.07.2019 20:30

Mathematics, 14.07.2019 20:30

Mathematics, 14.07.2019 20:30



Mathematics, 14.07.2019 20:30



Mathematics, 14.07.2019 20:30