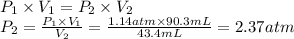Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, rightstrong9827
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 17:00, brandiwingard
What is the mass of phosphorous in a 51-kg person
Answers: 1

Chemistry, 22.06.2019 18:00, ambarpena14
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
You know the right answer?
The volume of ammonia gas at 1.14 atm of pressure is gradually decreased from 90.3 mL to 43.4 mL. Wh...
Questions in other subjects:

Mathematics, 19.03.2021 05:10

Physics, 19.03.2021 05:10



Mathematics, 19.03.2021 05:10


French, 19.03.2021 05:10

Mathematics, 19.03.2021 05:10


Physics, 19.03.2021 05:10