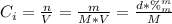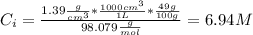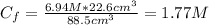Chemistry, 23.07.2020 03:01 hncriciacheichi
A solution of 49.0% H2SO4 by mass has a density of 1.39 g cm−3 at 293 K. A 22.6 cm3 sample of this solution is mixed with enough water to increase the volume of the solution to 88.5 cm3 . Find the molarity of sulfuric acid in this solution.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, flowergirly34
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 21.06.2019 23:00, carter1809
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 21:30, starl0rd211
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
A solution of 49.0% H2SO4 by mass has a density of 1.39 g cm−3 at 293 K. A 22.6 cm3 sample of this s...
Questions in other subjects:

History, 02.12.2020 14:00

Mathematics, 02.12.2020 14:00

Biology, 02.12.2020 14:00


Mathematics, 02.12.2020 14:10

Mathematics, 02.12.2020 14:10

Mathematics, 02.12.2020 14:10


Social Studies, 02.12.2020 14:10

Mathematics, 02.12.2020 14:10

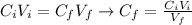
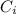 : is the initial concentration of the acid
: is the initial concentration of the acid 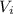 : is the initial volume of the solution = 22.6 cm³
: is the initial volume of the solution = 22.6 cm³ 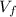 : is the final volume of the solution = 88.5 cm³
: is the final volume of the solution = 88.5 cm³