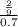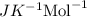Chemistry, 22.07.2020 23:01 MidnightAIY179
The osmotic pressure of a solution is calculated using the formula Π=MRT where Π is the osmotic pressure in atm, M is the molarity, R is the ideal gas constant, and T is the kelvin temperature. Part A What is the osmotic pressure of a solution made by dissolving 40.0 g of glucose, C6H12O6, in enough water to form 700.0 mL of solution at 37.0 ∘C ? Express your answer to three significant figures and include the appropriate units. nothing nothing


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mrylenastewart
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 05:50, aylengarcia090
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2


Chemistry, 22.06.2019 21:00, rhondafits9000
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
The osmotic pressure of a solution is calculated using the formula Π=MRT where Π is the osmotic pres...
Questions in other subjects:



Mathematics, 12.02.2021 14:00

History, 12.02.2021 14:00

English, 12.02.2021 14:00

Mathematics, 12.02.2021 14:00




Mathematics, 12.02.2021 14:00

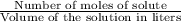
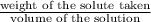

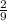 moles
moles