Chemistry, 19.07.2020 17:01 Aurionna101
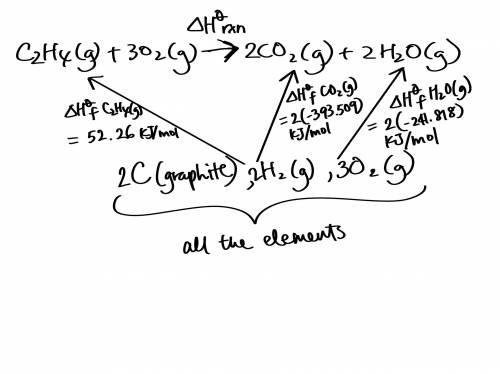
Use the standard enthalpies of formation for the reactants and products to solve for the ΔHrxn for the following reaction. (The ΔHf of C2H4 is 52.26 kJ/mol, CO2 is -393.509 kJ/mol, and H2O is -241.818 kJ.)
C2H4 (g) + 3O2(g) 2CO2 (g) + 2H2O(g)
ΔHrxn = (-345.64 kJ, -583.07 kJ, or -1,322.91 kJ).
The reaction is: (Endothermic or Exothermic).

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, momof7hardings
When would a bouncy ball have the most potential energy
Answers: 2

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3

Chemistry, 22.06.2019 19:30, dorindaramirez0531
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
Use the standard enthalpies of formation for the reactants and products to solve for the ΔHrxn for t...
Questions in other subjects:

History, 09.06.2021 19:00



Mathematics, 09.06.2021 19:00



Mathematics, 09.06.2021 19:00

Social Studies, 09.06.2021 19:00

Mathematics, 09.06.2021 19:00

Mathematics, 09.06.2021 19:00