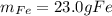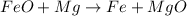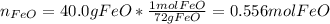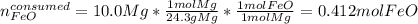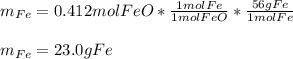Chemistry, 20.07.2020 01:01 gardinerr410
If 40.0 g of molten iron(II) oxide reacts with 10.0 g of mag-nesium, what is the mass of iron produced

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
If 40.0 g of molten iron(II) oxide reacts with 10.0 g of mag-nesium, what is the mass of iron produc...
Questions in other subjects:


Mathematics, 30.06.2021 03:20


Mathematics, 30.06.2021 03:20

Mathematics, 30.06.2021 03:20





Chemistry, 30.06.2021 03:20