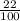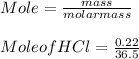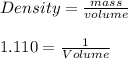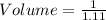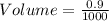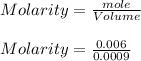Chemistry, 17.07.2020 19:01 GreenHerbz206
The mass percentage of hydrochloric acid within a solution is 22.00%. Given that the density of this solution is 1.110 g/mL, find the molarity of the solution.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:30, sweaversw9602
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 01:00, Angelofpink1143
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1

Chemistry, 23.06.2019 07:00, jennnifercrd59jc
Choose the correct statement about licensed veterinarians in the united states. a. they must be certified by the avma. b. they can treat all nonhuman animals. c. they can can treat only animals specified on the license. d. they must choose a specialty.
Answers: 2
You know the right answer?
The mass percentage of hydrochloric acid within a solution is 22.00%. Given that the density of this...
Questions in other subjects:

Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

English, 22.10.2020 01:01



Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01