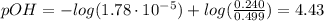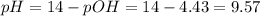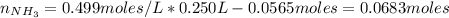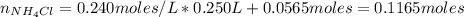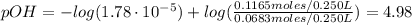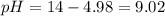Chemistry, 18.07.2020 01:01 DESIRE44030
A buffer solution contains 0.240 M ammonium chloride and 0.499 M ammonia. If 0.0565 moles of perchloric acid are added to 250 mL of this buffer, what is the pH of the resulting solution? (Assume that the volume does not change upon adding perchloric acid.)

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, fgcherubin
What happens to the atomic radius when an elctron is lost
Answers: 1


Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1
You know the right answer?
A buffer solution contains 0.240 M ammonium chloride and 0.499 M ammonia. If 0.0565 moles of perchlo...
Questions in other subjects:

Mathematics, 14.11.2019 10:31


Mathematics, 14.11.2019 10:31

History, 14.11.2019 10:31



Mathematics, 14.11.2019 10:31



![pOH = pKb + log(\frac{[NH_{4}Cl]}{[NH_{3}]})](/tpl/images/0708/9694/eb1d2.png)