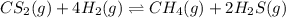Chemistry, 17.07.2020 19:01 haleynicole351ovewbg
The reaction system
CS2(g) + 4H2(g) CH4(g) + 2H2S(g)
is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of hydrogen is doubled?
a. As equilibrium is reestablished, the partial pressure of methane, CH4, decreases.
b. As equilibrium is reestablished, the partial pressure of carbon disulfide increases.
c. As equilibrium is reestablished, all the partial pressures will decrease.
d. As equilibrium is reestablished, the partial pressure of hydrogen sulfide decreases.
e. As equilibrium is reestablished, the partial pressure of hydrogen decreases.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, wizz4865
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3


Chemistry, 22.06.2019 13:00, rome58
Lab reagent, hypothesis test. a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl. these six measurements are assumed to be an srs of all possible measurements from solution. they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution. carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1
You know the right answer?
The reaction system
CS2(g) + 4H2(g) CH4(g) + 2H2S(g)
is at equilibrium. Which of the followi...
is at equilibrium. Which of the followi...
Questions in other subjects:

Mathematics, 16.10.2020 17:01

History, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01

English, 16.10.2020 17:01

English, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01