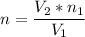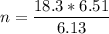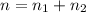Chemistry, 16.07.2020 17:01 silasjob09
A flexible container at an initial volume of 6.13 L contains 6.51 mol of gas. More gas is then added to the container until it reaches a final volume of 18.3 L. Assuming the pressure and temperature of the gas remain constant, calculate the number of moles of gas added to the container.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, milkshakegrande101
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 16:00, bbrogle5154
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
A flexible container at an initial volume of 6.13 L contains 6.51 mol of gas. More gas is then added...
Questions in other subjects:

Mathematics, 09.06.2020 21:57

Mathematics, 09.06.2020 21:57

Mathematics, 09.06.2020 21:57


Biology, 09.06.2020 21:57


Biology, 09.06.2020 21:57



Mathematics, 09.06.2020 21:57