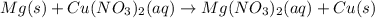When a strip of magnesium metal is placed in an aqueous solution of copper(II) nitrate, elemental copper coats the surface of the magnesium strip and aqueous magnesium nitrate forms.
1. As a reactant, what is the charge on copper?
2. As a product, what is the charge on copper?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:00, lwattsstudent
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2
You know the right answer?
When a strip of magnesium metal is placed in an aqueous solution of copper(II) nitrate, elemental co...
Questions in other subjects:


Mathematics, 22.07.2021 14:00

Physics, 22.07.2021 14:00

English, 22.07.2021 14:00

Mathematics, 22.07.2021 14:00

Mathematics, 22.07.2021 14:00



Mathematics, 22.07.2021 14:00

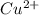 ).
).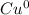 ).
).