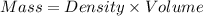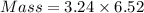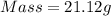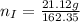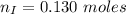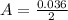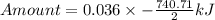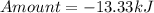Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

You know the right answer?
How much heat is liberated at constant pressure when 1.41 g of potassium metal reacts with 6.52 mL o...
Questions in other subjects:

English, 18.07.2019 09:00

Physics, 18.07.2019 09:00

Mathematics, 18.07.2019 09:00




Mathematics, 18.07.2019 09:00

History, 18.07.2019 09:00


Computers and Technology, 18.07.2019 09:00

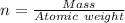
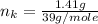 ---where 39 is the atomic weight of potassium
---where 39 is the atomic weight of potassium