
Chemistry, 13.10.2019 23:50 ANONYMUSNESS8670
The sodium atom loses an electron to form a sodium ion (na+). which statement is correct with respect to its atomic radius?
the sodium ion has a larger radius than the atom.
the sodium ion has a smaller radius than the atom.
the sodium ion and the sodium atom radii are the same size.
the sodium ion has twice the radius of the sodium atom.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, only1cache
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3


Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1
You know the right answer?
The sodium atom loses an electron to form a sodium ion (na+). which statement is correct with respec...
Questions in other subjects:



SAT, 11.03.2020 23:24



Mathematics, 11.03.2020 23:24



Chemistry, 11.03.2020 23:24

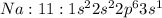
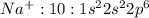
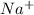 has 10 electrons and 11 protons. Now 11 protons present in the nucleus can easily influence 10 electrons towards itself, the effective nuclear charge increases, the valence electrons are more tightly held by the nucleus and thus the size decreases.
has 10 electrons and 11 protons. Now 11 protons present in the nucleus can easily influence 10 electrons towards itself, the effective nuclear charge increases, the valence electrons are more tightly held by the nucleus and thus the size decreases.

