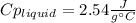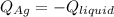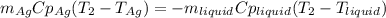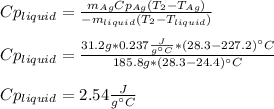Chemistry, 16.07.2020 01:01 makaylaf9479
3. A 31.2-g piece of silver (s = 0.237 J/(g · °C)), initially at 277.2°C, is added to 185.8 g of a liquid, initially at 24.4°C, in an insulated container. The final temperature of the metal–liquid mixture at equilibrium is 28.3°C. What is the specific heat of the liquid? Neglect the heat capacity of the container.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, issachickadi
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2

Chemistry, 23.06.2019 02:30, ineedhelp2285
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2
You know the right answer?
3. A 31.2-g piece of silver (s = 0.237 J/(g · °C)), initially at 277.2°C, is added to 185.8 g of a l...
Questions in other subjects:



Mathematics, 11.02.2021 07:50

Biology, 11.02.2021 07:50




Chemistry, 11.02.2021 07:50


Mathematics, 11.02.2021 07:50