
Chemistry, 15.07.2020 01:01 michellemunoz250
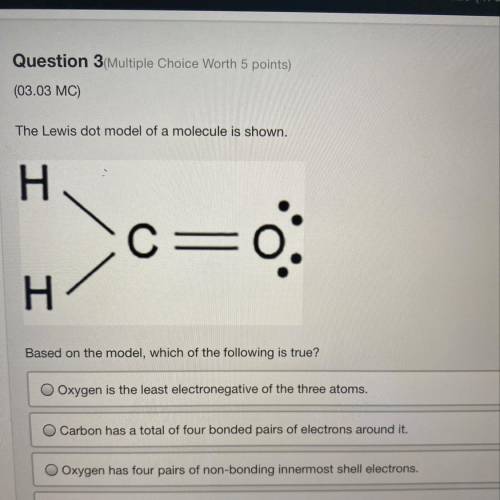
The Lewis dot model of a molecule is shown.
Based on the model, which of the following is true?
O Oxygen is the least electronegative of the three atoms.
Carbon has a total of four bonded pairs of electrons around it.
O Oxygen has four pairs of non-bonding innermost shell electrons.
Carbon has an incomplete octet as it transfers an electron to each hydrogen.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ciarakelly636owuiup
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 22.06.2019 09:00, angelrenee2000
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2


Chemistry, 23.06.2019 06:10, jamesgotqui6
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a. what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2
You know the right answer?
The Lewis dot model of a molecule is shown.
Based on the model, which of the following is true?
Questions in other subjects:


Mathematics, 16.04.2020 02:24








English, 16.04.2020 02:24



