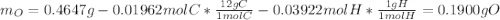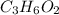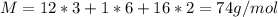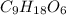Chemistry, 15.07.2020 22:01 emilymendes546
A 0.4647-g sample of a compound known to contain only carbon, hydrogen, and oxygen was burned in oxygen to yield 0.01962 mol of CO2 and 0.01961 mol of H2O. The empirical formula of the compound was found to be C3H6O2. Show how this was calculated. What does the empirical formula tell you about the compound? The molar mass of the actual compound was found to be 222.27g/mol. Find the molecular formula of this compound. What does the molecular formula tell you about the compound? Can you see what type of functional group this compound could have?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, tristen2001
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1


Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
A 0.4647-g sample of a compound known to contain only carbon, hydrogen, and oxygen was burned in oxy...
Questions in other subjects:

English, 22.02.2021 15:40

Social Studies, 22.02.2021 15:40

History, 22.02.2021 15:40

Mathematics, 22.02.2021 15:40

Mathematics, 22.02.2021 15:40


Chemistry, 22.02.2021 15:40

Social Studies, 22.02.2021 15:40

Mathematics, 22.02.2021 15:40