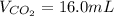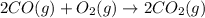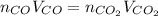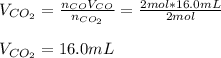Chemistry, 15.07.2020 09:01 westlakebuddy1229
Assuming the same temperature and pressure for each gas, how many milliliters of carbon dioxide are produced from 16 0 mL of CO
2 CO(g) + O2(g) 4, 2 CO2 (g)
Express your answer with the appropriate units.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:40, yfgkeyonna
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 12:00, ctyrector
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1
You know the right answer?
Assuming the same temperature and pressure for each gas, how many milliliters of carbon dioxide are...
Questions in other subjects:





English, 15.10.2020 04:01



Mathematics, 15.10.2020 04:01


Mathematics, 15.10.2020 04:01