Chemistry, 15.07.2020 04:01 Lydiaxqueen
When 100.0 mL of 0.40 M of HF and 100.0 mL of 0.40 M of NaOH are mixed, the resulting mixture is:a. a strong acid b. a strong base c. a weak acid d. a weak base e. a buffer

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
When 100.0 mL of 0.40 M of HF and 100.0 mL of 0.40 M of NaOH are mixed, the resulting mixture is:a....
Questions in other subjects:

Mathematics, 24.03.2020 22:56

Spanish, 24.03.2020 22:56





Mathematics, 24.03.2020 22:56



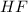 and
and 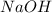 . So:
. So: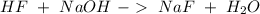
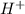
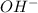
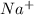 and the anion is
and the anion is