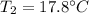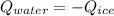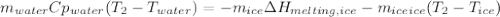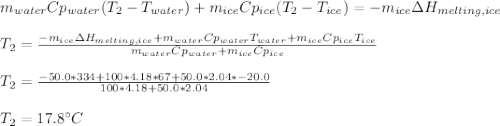Chemistry, 15.07.2020 04:01 alejandraluna95
You add 50.0 g of ice initially at ‒20.0 °C to 1.00 x 102 mL warm water at 67.0 °C. When all the ice melts, the water temperature is found to be somewhere above 0 °C. Calculate the final temperature of the water.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, lydiadmanautou04
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 09:00, boxergirl2062
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 17:30, rollercoasterbuddies
Why is the melting of ice a physical change ?
Answers: 1

Chemistry, 23.06.2019 03:00, cabreradesirae4807
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2
You know the right answer?
You add 50.0 g of ice initially at ‒20.0 °C to 1.00 x 102 mL warm water at 67.0 °C. When all the ice...
Questions in other subjects:

Mathematics, 06.01.2021 17:30


Mathematics, 06.01.2021 17:30

Social Studies, 06.01.2021 17:30

English, 06.01.2021 17:30


Mathematics, 06.01.2021 17:30


SAT, 06.01.2021 17:30