Chemistry, 15.07.2020 01:01 whocares1234
calculate atomic weight of silver which has two isotopes with the following properties : silver-107 (106.91 amu, 51.84% natural occurence) and silver-109 (108.90 amu, 48.16% natural occurence). please help

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 22:00, notearslefttocry14
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
calculate atomic weight of silver which has two isotopes with the following properties : silver-107...
Questions in other subjects:

Mathematics, 20.01.2021 03:40

Mathematics, 20.01.2021 03:40

Physics, 20.01.2021 03:40



Mathematics, 20.01.2021 03:40

Mathematics, 20.01.2021 03:40

Mathematics, 20.01.2021 03:40

Mathematics, 20.01.2021 03:40

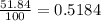
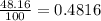
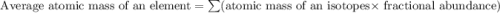
![A=\sum[(106.91\times 0.5184)+(108.90\times 0.4816)]](/tpl/images/0706/2294/732da.png)