
Chemistry, 15.07.2020 01:01 andersonrocksc
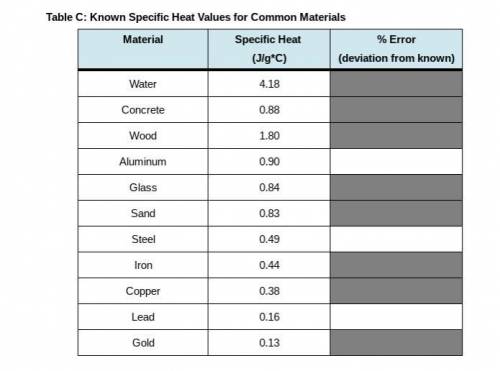
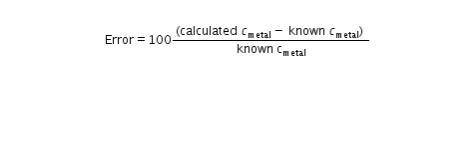
PLEASE I NEED HELP WITH THE BOXES ASAP !! in this last step, return to Step 10 in your Lab Guide to calculate the error between your calculated specific heat of each metal and the known values in Table C. Follow the directions given in your Lab Guide, using this formula: Error = 100 times StartFraction calculated c subscript metal minus known c subscript metal over known C subscript metal EndFraction.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, drivinghydra
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 06:30, 91miketaylor
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1

Chemistry, 22.06.2019 18:00, jessicannoh5965
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
PLEASE I NEED HELP WITH THE BOXES ASAP !! in this last step, return to Step 10 in your Lab Guide to...
Questions in other subjects:





Mathematics, 28.06.2019 13:30

Mathematics, 28.06.2019 13:30


Mathematics, 28.06.2019 13:30

Mathematics, 28.06.2019 13:30





