
Chemistry, 14.07.2020 01:01 Prettygirlyaya
The equilibrium constant in terms of pressures for the reaction COBr2(g) CO(g) + Br2(g) is Kp = 5.40 at 346 K. (a) A pure sample of gaseous carbonyl bromide, COBr2(g), is introduced into a rigid flask at a temperature of 346 K so that its original pressure is 0.123 atm. Calculate the fraction of this starting material that is converted to products at equilibrium.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, Queenquestion5967
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3


Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 10:00, JOEFRESH10
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1
You know the right answer?
The equilibrium constant in terms of pressures for the reaction COBr2(g) CO(g) + Br2(g) is Kp = 5.40...
Questions in other subjects:


Mathematics, 05.05.2020 07:29

English, 05.05.2020 07:29

Mathematics, 05.05.2020 07:29

Spanish, 05.05.2020 07:29

Mathematics, 05.05.2020 07:29

Mathematics, 05.05.2020 07:29

History, 05.05.2020 07:29


Mathematics, 05.05.2020 07:29

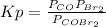 = 5.40
= 5.40

