
Chemistry, 14.07.2020 01:01 laurachealsy923
Calculate Ecell at 80 ºC for a voltaic cell based on the following redox reaction: H2(g, 1.25 atm) + 2AgCl(s) → 2Ag(s) + 2H+(aq, 0.10 M) + 2Cl–(aq, 1.5 M) The standard cell potential Eºcell = +0.18 V at this temperature.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
Calculate Ecell at 80 ºC for a voltaic cell based on the following redox reaction: H2(g, 1.25 atm) +...
Questions in other subjects:

Mathematics, 14.01.2021 03:20

Mathematics, 14.01.2021 03:20

Biology, 14.01.2021 03:20

Social Studies, 14.01.2021 03:20

Computers and Technology, 14.01.2021 03:20


Mathematics, 14.01.2021 03:20


Social Studies, 14.01.2021 03:20

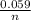 logQ
logQ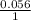 log 0.067
log 0.067

