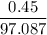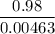Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2

You know the right answer?
If 0.98 g of an unknown was dissolved in 10.30 g of solvent and the resulting solution has a molalit...
Questions in other subjects:

Mathematics, 11.06.2020 23:57



English, 11.06.2020 23:57


Mathematics, 11.06.2020 23:57


Health, 11.06.2020 23:57

Biology, 11.06.2020 23:57

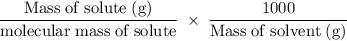 ......(i)
......(i)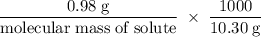
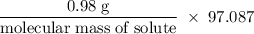
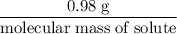 =
=